
"Business opportunity with high growth potential"
"The expansion of commercial manufacturing capabilities is intended to complement the Company's in-house drug development programs and provide additional high-value services to third-party customers preparing for commercialization. The contract signed today is the second such agreement in the past twelve months. We generally view this type of contract activity as a significant business opportunity with high growth potential," said CEO Hugh Rogers. In addition to a formula for coating enteric-coated tablets, which the Company acquired just a few weeks ago, BioNxt also has a technology for delivering the Parkinson's drug rotigotine via patches. A comparative study of an established product had produced almost identical results last fall. At that time, the Company was confident that it could level out any remaining differences in the next stage of development.
Great market potential for active ingredient patches
The recent market success reported by BioNxt could be just the beginning for the Company. With its own active ingredient patches, including the proven active ingredient rotigotine, BioNxt could soon be marketing another promising product. A recent court ruling in the US suggests that there could soon be movement around this product. The US Court of Appeals has upheld an earlier ruling by a federal court in Delaware that the so-called '589 patent for the well-known Parkinson's patch Neupro is invalid. The ruling favors generic manufacturers who want to make copycat preparations for Neupro and could be the starting signal for the commercialization of the active ingredient patch developed by BioNxt's German subsidiary Vektor Pharma TF.
the market for rotigotine patches could grow to USD 766 million by 2030.
Market researchers at Wissen Market Research estimate that total global sales of rotigotine patches were USD 518 million in 2021 and will grow to more than USD 766 million by 2030. One of the most important markets is the US, where a court has now given the go-ahead for generics in this area. In the context of the transdermal drug patch that BioNxt subsidiary Vektor Pharma TF has developed for use with rotigotine, its versatility is also important. In principle, the technology can deliver all drugs that need to be slowly and evenly released into the body, such as painkillers or other medications for chronic conditions.
Market barely prices in operational successes so far
The third business segment of active psychoactive ingredients, which has received little attention in recent months, could also benefit from innovative dosage forms, as the correct dosage is more important than ever here. In recent years, various studies have demonstrated positive effects of psychoactive substances in chronic diseases and have triggered at least an initial rethink among regulators. Despite the existing growth prospects in this area, the opportunities for BioNxt with already approved active ingredients on the generics market will likely outweigh the risks, at least in the short and medium term.
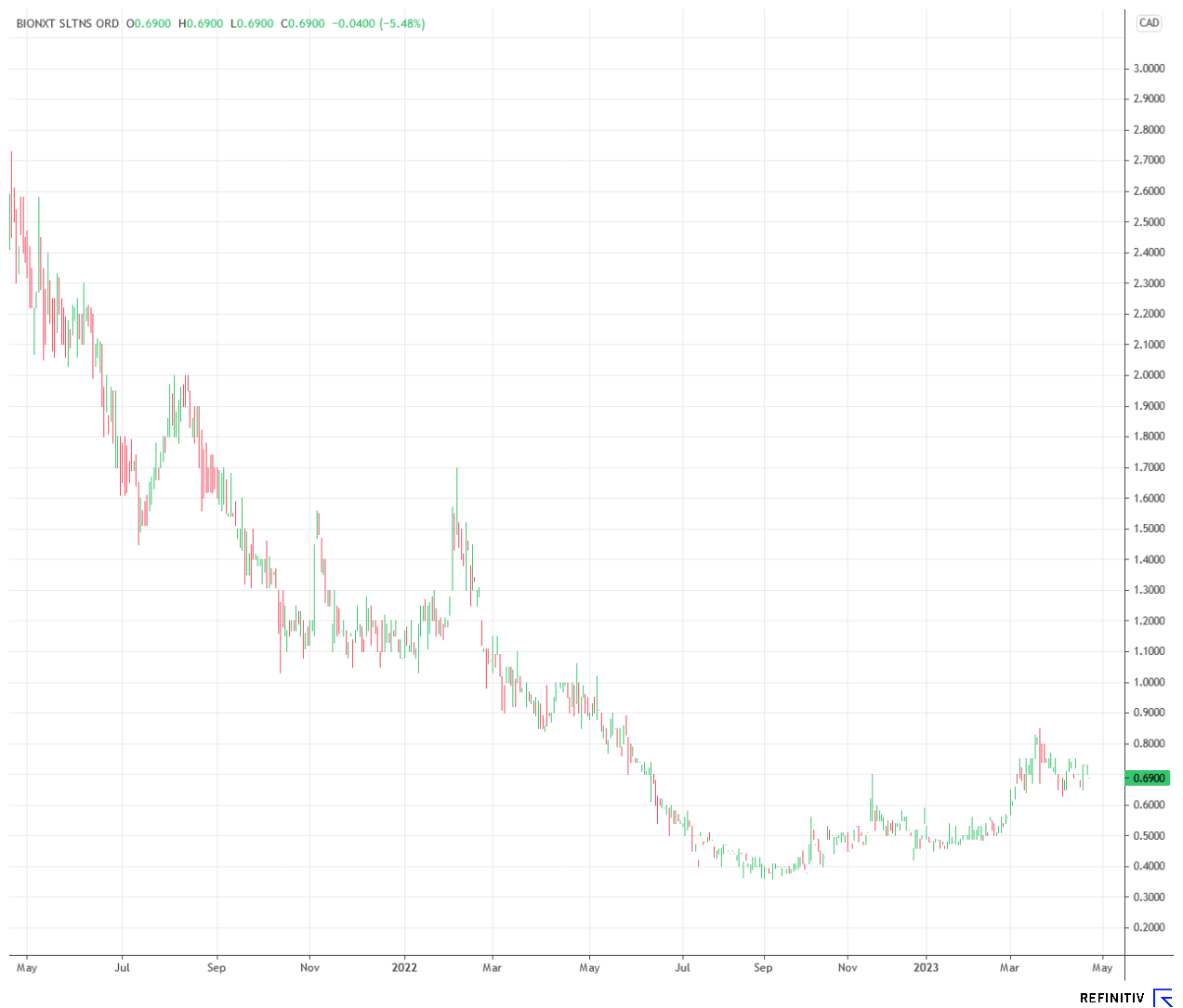
Following the landmark ruling around patent protection for rotigotine patches in the US and the existing letter of intent for the production of biosensor products for testing oral diseases from last fall, BioNxt has taken another important step towards the commercialization of its products with the recently announced manufacturing agreement with an international pharmaceutical company. Looking at the BioNxt share price performance, these successes are barely priced in so far: The share is still trading far below its level of a year ago. However, as soon as the letters of intent and contracts are reflected in concrete figures, this is likely to change.
Interim conclusion: Rotigotine patch as the "next big thing" for BioNxt?
The Canadian-German biotech company BioNxt is forming partnerships and entering into business relationships. Thanks to BioNxt's platform approach, these newly established business relationships could also bear fruit in other areas. With Rotigotine patches already competitive during initial comparative testing last fall, BioNxt also has another potential blockbuster already in its portfolio. It is quite possible that potential business partners first wanted to wait for the pending appeal around the patent lawsuit against the Parkinson's patch Neupro and are now contacting suitable partners. The coming months will be exciting for BioNxt shareholders.
The update is based on the initial report 02/2022

