The Canadian biotech company Defence Therapeutics has moved closer to its goal of bringing several projects in the fight against cancer into clinical trials in 2023. Around the AccuTOX™ chemotherapy project, the Company announced a collaboration with the renowned City of Hope National Medical Center in Duarte in the Los Angeles area. The clinic is considered one of the best cancer clinics in the US and worldwide. The affiliated Beckman Research Institute was founded in 1951 and is synonymous with medical breakthroughs in cancer, HIV and diabetes that directly benefit patients, true to the motto of translational research "from bench to bedside".

City of Hope: "World leader in clinical trials."
Measured by citations, the Beckman Research Institute ranks 481 out of 20331 universities worldwide. Citations are like a kind of currency within the scientific community and indicate how relevant a research contribution is. The Beckman Research Institute and the affiliated City of Hope National Medical Center are now helping Defence Therapeutics advance its AccuTOX™ compound to regulatory approval. The experienced scientists prepare all applications for a Phase I study, take care of study design and review all preliminary work already done by Defence Therapeutics in order to be able to initiate a Phase I study with AccuTOX™ as soon as possible.
submitted by City of Hope Hospital annually.
"This is an important milestone, and we are very confident that City of Hope will support us in this FDA process to approve the initiation of a Phase I clinical trial in the US. With AccuTOX™ potentially entering clinical trials in the US later this year, Defence expects to be able to use AccuTOX™ for the treatment of various cancer tumors," said Sébastien Plouffe, CEO and President of Defence. The City of Hope National Medical Center and affiliated research facilities implement approximately 400 clinical trials involving over 6,000 patients annually and consider themselves "world leaders in the conduct of clinical trials." For the progress of Defence Therapeutics and its chemotherapy project AccuTOX™, the university hospital should be the perfect partner - in addition to preparing the registration documents, the hospital could also conduct the Phase I trial in the next step - experience around cancer studies as well as suitable patients are sufficiently available at the City of Hope, which invested around USD 1 billion in a "Cancer Campus" as recently as 2019.

AccuTOX™ as one project among many
Also, partnering with such a renowned university hospital should ensure that Defence Therapeutics' projects are even more widely recognized within the scientific community. Defence Therapeutics' other projects, such as mRNA vaccines against various forms of cancer, Accum™ as an ADC drug booster or the planned protein-based vaccine against HPV - a successful Phase I trial application should provide a tailwind for all of its projects. More recently, Defence Therapeutics partnered with the University of Montréal and the Lady Davis Institute of the Jewish General Hospital to launch a new vaccine platform sponsored by Canadian biotech catalyst CQDM, whose oversight boards include representatives from academia and pharmaceutical companies. The now-announced collaboration with the City of Hope Medical Center, the seventh-best cancer hospital in the US, is thus already the second high-profile cooperation with a renowned research institution within a few weeks.
At the same time, Defence Therapeutics is presenting itself to investors and presenting its comprehensive portfolio to interested representatives from the business world. At the beginning of February, the Company presented at Immuno-Oncology 360° in New York and a little later online at the 6th International Investment Forum (IIF). The presentation by Head of Research Prof. Dr Moutih Rafei is available online and provides an overview of the Company's activities in just a few minutes.
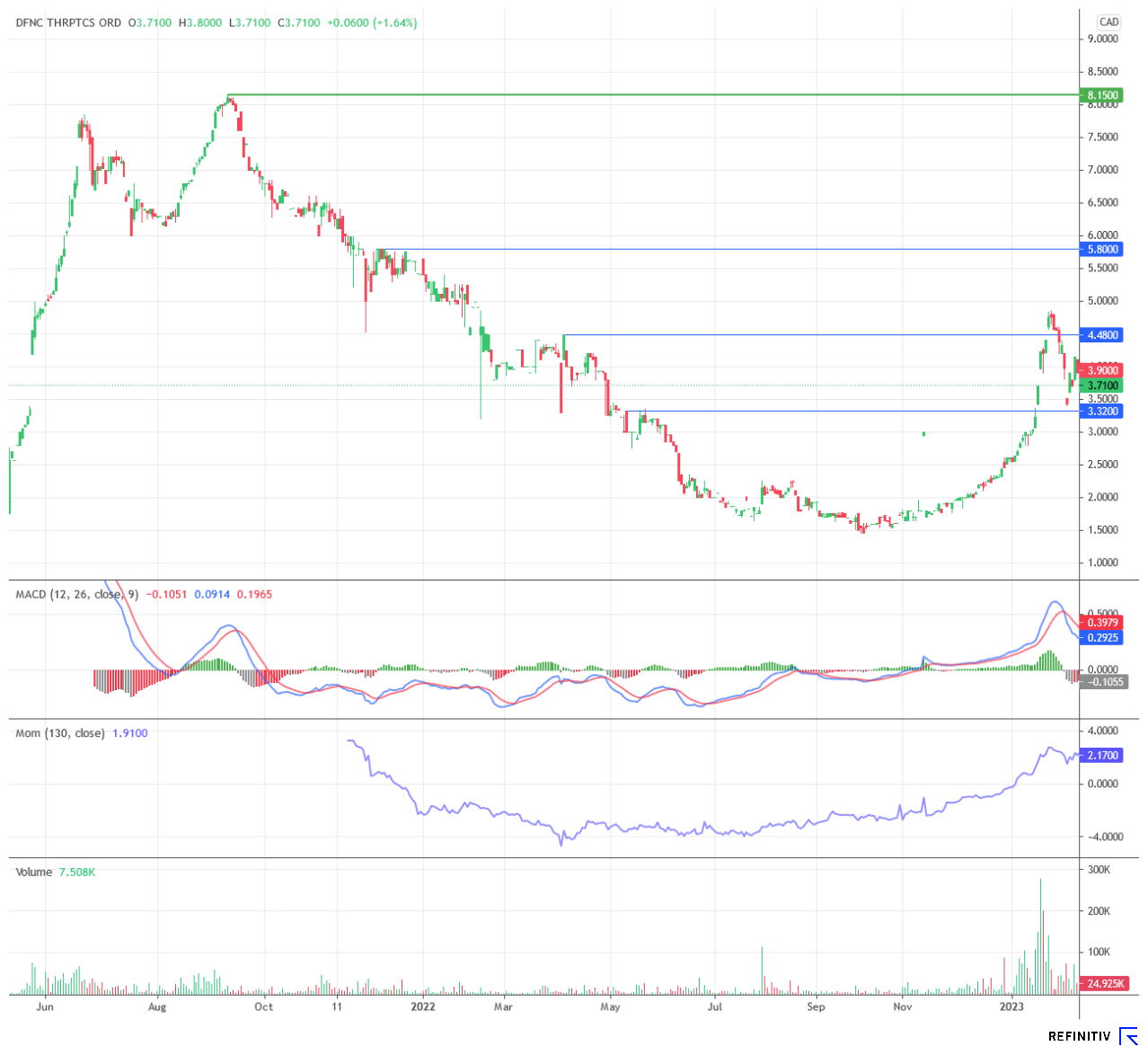
Chart technical situation
Technically, the shares of Defence Therapeutics continue to be on the upswing. After a dynamic price rally in January, the stock entered a consolidation movement but is holding exceedingly stable at the current level. In view of the takeover fantasy noted by several analysts in the biotech sector, as well as the numerous projects that Defence Therapeutics intends to advance in parallel in 2023, the share is always good for building up new momentum. Even the previous all-time high of CAD 8.15 is within the realm of possibility - provided operational progress is made. The most recent lows offer themselves as hedging opportunities.
Interim conclusion: Strong partners for strong projects
The now-announced collaboration of Defence Therapeutics with the City of Hope National Medical Center underlines the ambitions of the versatile biotech from Canada. The researchers from the greater Los Angeles area oversee numerous studies around active substances against cancer every year and bring everything they need to have a Phase I study approved by the regulatory authority FDA. The reputation of the university hospital and the associated Beckman Research Institute also increases the visibility of all other Defence Therapeutics projects and, at the same time, reduces the risk of procedural errors, which can never be completely ruled out in complex approval processes.
The shares of Defence Therapeutics have come to rest after their dynamic rise in January - the overall chart picture nevertheless remains promising. Given Defence Therapeutics' operational progress and its bulging and synergistic pipeline thanks to its platform approach, investors can expect a continuous news flow. According to management, the Company has received positive feedback during recent investor events. The stock remains an exciting alternative in the biotech sector, which is currently in high demand.
The update is based on the initial report 12/2021

