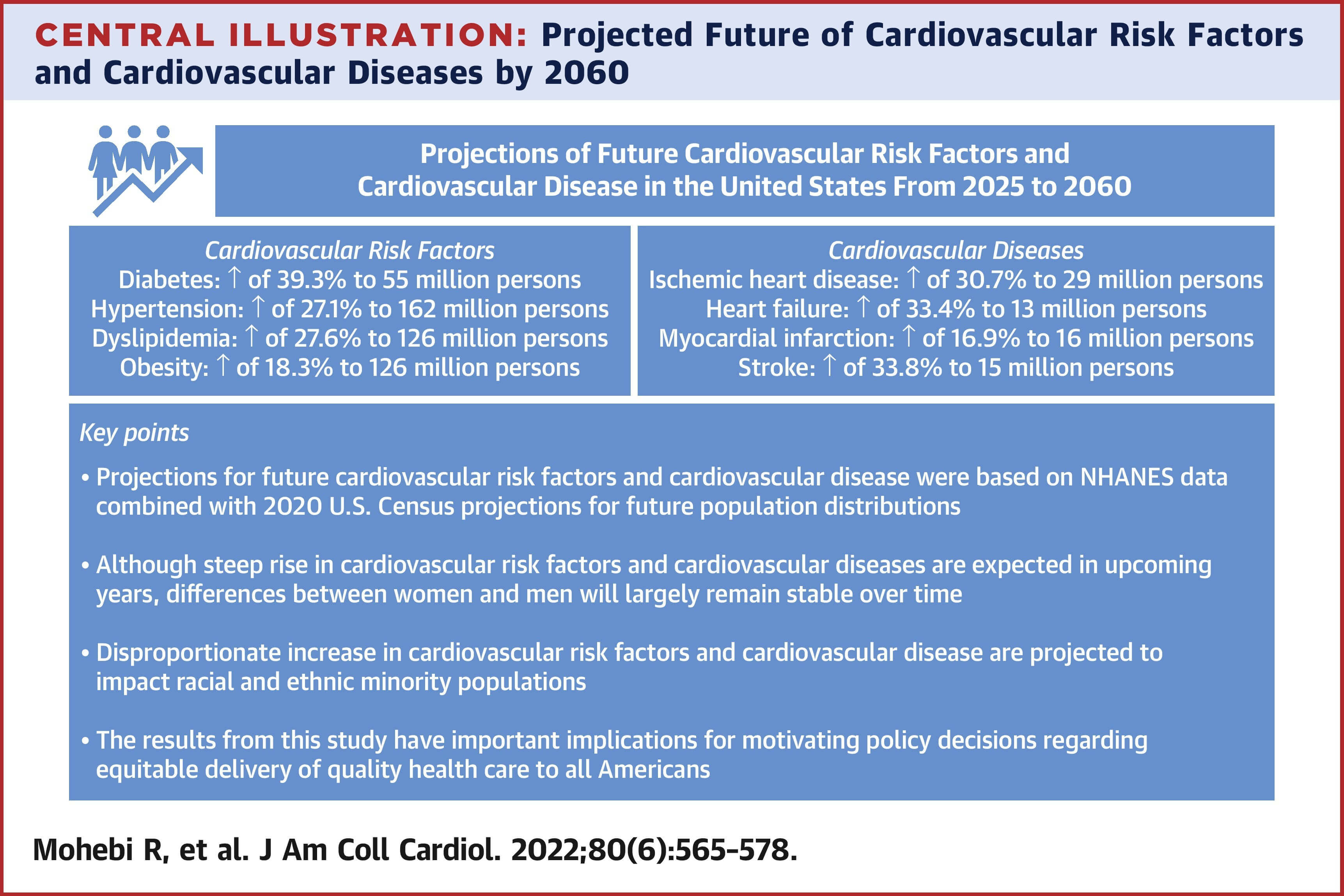
Over half a billion people worldwide are affected by cardiovascular disease. Whether heart attack, stroke or inflammation of the heart: in 2021, cardiovascular diseases led to 20.5 million deaths worldwide1 . This means that heart disease is responsible for around a third of all deaths worldwide. Experts believe that up to 80% of premature heart attacks and strokes could be prevented. However, it is precisely the means of diagnosis, prevention, and treatment that do not reach the people who need it most.
About 4 out of 5 deaths from cardiovascular disease occur in low and middle-income countries. There is also a slight year-over-year increase in deaths in the US.2
The market opportunity for Cardiol Therapeutics
US researchers predict that the number of heart diseases will increase significantly by 2060. 3 The reasons for this are lack of exercise, unhealthy diet, and stress. When accumulated, these factors lead to high blood pressure, diabetes, and inflammatory processes in the body. The research team led by James L. Januzzi from Massachusetts General Hospital and Harvard Medical School in Boston came to the same conclusion as the World Heart Federation: in future, people who cannot afford comprehensive healthcare will be particularly affected by heart disease.
The life sciences company Cardiol Therapeutics will fill this gaping hole in the future with its promising drugs. The Canadian company, based in Ontario, focuses on the research and clinical development of anti-inflammatory and anti-fibrotic therapies for the treatment of heart disease. The drug CardiolRx™ (cannabidiol) is to be administered as an oral solution to patients with heart disease. CardiolRx™ is already recognized by the US Food and Drug Administration (FDA) as an orphan drug for treating pericarditis, which includes recurrent pericarditis. In the European Union, a disease is classified as an orphan disease if fewer than 5 in 10,000 people suffer from it. 4

Because that there are over 6,000 different rare diseases and around 250 new ones are added every year, the total number of people affected is high despite the rarity of each individual disease. Orphan drugs are permitted when no alternative therapies or cures are on the market. CardiolRx™ has achieved this status. For the market in the US 5 this means seven years of exclusivity for Cardiol Therapeutics, for the European market even ten years. Given the increase in cases of heart disease and the lack of alternative adequate therapies, investors can analyze the market opportunity for Cardiol Therapeutics with razor-sharp precision.
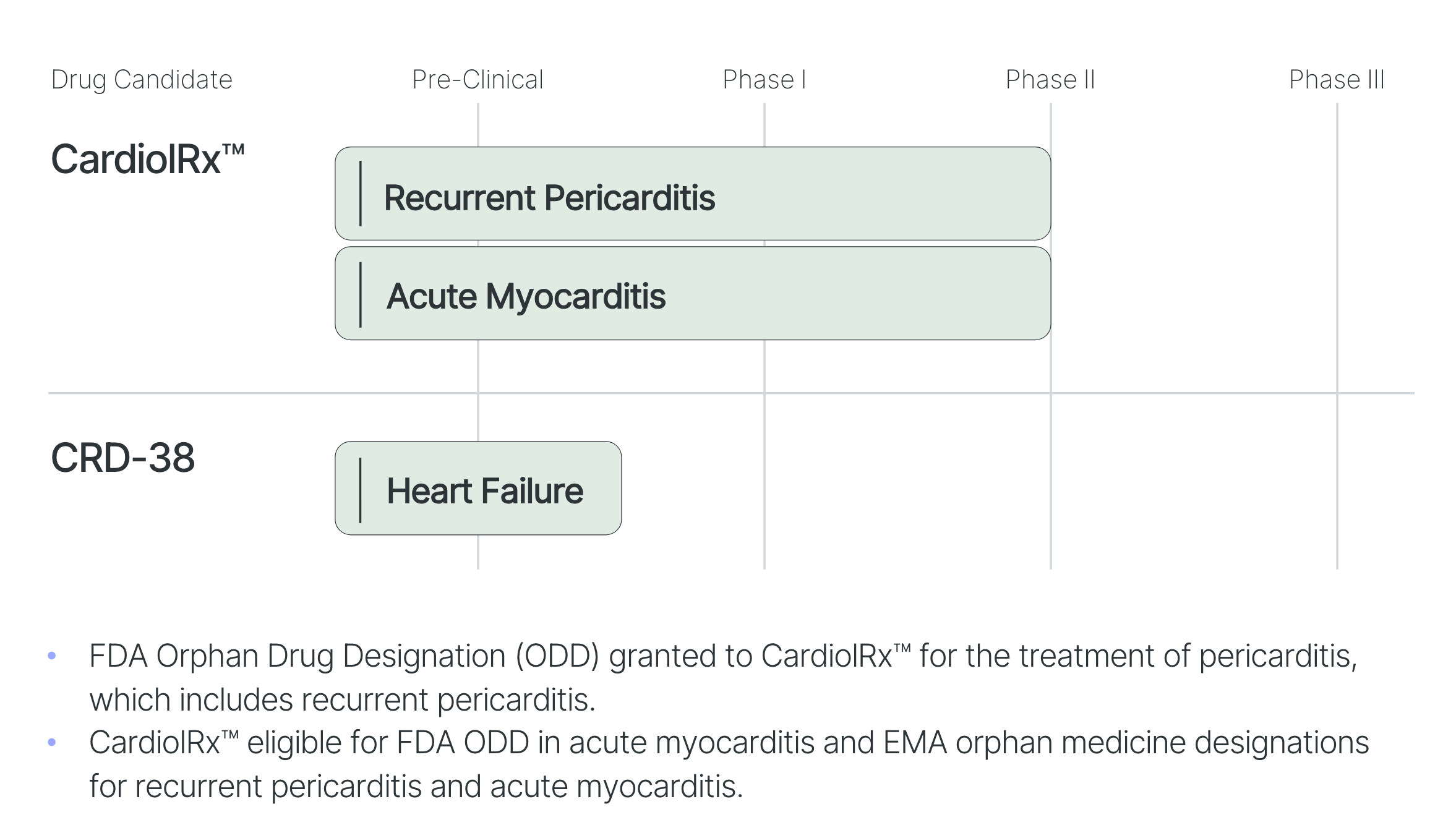
The cardiac diseases that the life sciences company will research and treat with its compounds include recurrent pericarditis, acute myocarditis, and heart failure. Pericarditis is an inflammation of the pericardium, the membrane surrounding and holding the heart within the chest. The disease leads to fluid accumulation and thickening of the tissue. Symptoms can include chest pain, shortness of breath, and depression, as sufferers are severely affected by the loss of physical performance. Severe cases can severely impact quality of life and physical activity. Emergency room visits or hospitalizations are then required. In the US, there are approximately 18,000 hospitalizations due to pericarditis annually, based on a rate of 5.4 per 100,000 population. The number of patients with recurrent pericarditis is 38,000 annually in the United States.
about 30% of patients suffer a relapse within 18 months, and 50% of these patients suffer further relapses.
Heart disease recurrent pericarditis: The gradual deterioration
Doctors diagnose recurrent pericarditis when symptoms reappear after an acute outbreak following a symptom-free period of at least 4-6 weeks. People affected by this disease often suffer for years. In the US, there are around 160,000 cases of pericarditis each year, 38,000 of which are associated with relapses. The average hospital costs amount to USD 20,000 to 30,000 for a stay of 6 to 9 days. Even if the average per capita wealth in the US is higher than in Europe, savings can quickly evaporate with such a disease progression. 6
Because the relapse rate is noteworthy: around 30% of those affected relapse again within 1.5 years, with up to 50% of them having further relapses. The average duration of recurrent pericarditis in difficult-to-treat patients is 4.7 to 6.2 years. One can calculate how this disease affects physical, mental, and financial health.
Remarkably positive results from the Phase II MAvERIC-Pilot study for recurrent pericarditis treatment excite experts
"We are particularly pleased to announce the primary endpoint data from the MAvERIC-Pilot study 7 . These data demonstrated that oral administration of our small molecule, CardiolRx™, significantly reduced pain and inflammation associated with pericarditis. The magnitude of this reduction was remarkably comparable to the changes reported following the administration of immunosuppressive therapy with biologics commonly used in third-line treatment of recurrent pericarditis," said David Elsley, President and CEO of Cardiol Therapeutics.

Cardiol Therapeutics is investigating the tolerability, safety, and efficacy of CardiolRx™ in patients with recurrent pericarditis in the open-label Phase II 'MAvERIC-Pilot' study. The study aims to assess improvements in objective measures of this rare disease. Cardiol has already reached the planned target size of 25 patients for the MAvERIC-Pilot study in the US in February 2024. This will enable the research team to publish high-quality clinical trial data for the medical community.
The MAvERIC-Pilot study included 27 patients diagnosed with symptomatic recurrent pericarditis. Each patient had a high burden of disease, as reflected by both a patient-reported pain score averaging 5.8 out of 10 at baseline and the number of previous episodes of pericarditis (9 patients or 33%, had 2 previous episodes, 9 patients, or 33% had 3 such episodes, 4 patients or 15% had 4 episodes, and 5 patients or 19% had more than 4 episodes).
The primary endpoint of the Phase II study is the change in patient-reported pericarditis pain at eight weeks, as measured by an 11-point numeric rating scale (NRS) that has been validated and applied in several clinical trials. The numeric rating scale (NRS) is a pain screening tool commonly used to assess pain severity by using a scale from 0 to 10, where zero is "no pain" and 10 is "the worst pain imaginable". Secondary endpoints include the NRS score after 26 weeks of treatment and changes in circulating levels of C-reactive protein (CRP), a commonly used marker of inflammation in patients with cardiovascular disease. C-reactive protein is found in blood plasma and its concentration increases when there is inflammation in the body.
The results in summary:
- The subjectively perceived pain of the pericarditis patients on the NRS scale decreased on average by 3.7 points, from 5.8 to 2.1 after 8 weeks.
- In 80% of patients with a CRP value ≥1 mg/dl, the CRP value normalized to ≤ 0.5 mg/dl after 8 weeks, with an average decrease from 5.7 mg/dl to 0.3 mg/dl.
- 89% of patients successfully transitioned from the treatment phase to the extension phase of the study, which included an additional 18-week treatment period with CardiolRx™.
- CardiolRx™ was found to be safe and well tolerated, with a positive clinical response in the pericarditis patients.
These results are outstanding and it is possible that Cardiol Therapeutics is already on the takeover list of major life sciences companies. One thing is certain: with such positive study results, nothing stands in the way of the upcoming Phase III study.
Previous treatment methods and their consequences
In the case of recurrent pericarditis, the usual treatment is primarily the administration of anti-inflammatory drugs such as NSAIDs or aspirin, possibly together with colchicine. In persistent cases, steroid hormones are used, although there are concerns about safety and discontinuation. The side effects of steroid hormones are severe: high blood pressure, muscle wasting and loss of calcium in the bones can occur, further affecting the body of affected patients.8 An FDA-approved therapy called Rilonacept costs over USD 150,000 per year and is used primarily for repeat cases if one has the savings to pay for it.
Cannabidiol (CBD) is a chemical compound found in hemp plants. The active ingredient has no psychoactive effect but has been and is being extensively researched for its medical use. Cannabidiol relieves pain, reduces inflammation, lowers anxiety, and improves sleep.
Starting signal for Phase III study with thousands of patients
Cardiol Therapeutics is committed to an evidence-based approach in the further development of CardiolRx™. Therefore, the team is expected to design a robust pivotal Phase III clinical trial in recurrent pericarditis to support regulatory approval of CardiolRx™. Researchers design Phase III studies to demonstrate whether a product provides a treatment benefit for a specific population. The Phase III study will also evaluate the absence of pericarditis recurrence, potentially demonstrating clinically relevant benefits early on. With only one FDA-approved therapy for recurrent pericarditis and CardiolRx™ having a strong safety profile compared to currently available anti-inflammatory and immunosuppressive drugs, the market niche that Cardiol can fill is enormous.
"Based on the clinically meaningful impact of CardiolRx™ on the key symptom of this highly debilitating disease, we now anticipate that the totality of the data from the MAvERIC-Pilot study will support progression to a Phase III study of CardiolRx™, which we hope will achieve our goal of providing thousands of pericarditis patients with a more accessible and non-immunosuppressive treatment option," says David Elsley.
The market for heart disease holds great potential, especially if researchers can demonstrate in their pivotal study that the recurrence of recurrent pericarditis is prevented. While it may not be technically accurate to speak of a cure, the possibility of preventing another outbreak is significant. The regained quality of life for patients is, in the truest sense of the word, invaluable.
Wall Street analysts set price target at USD 9 per share
Analysts at New York's prestigious investment bank H.C. Wainwright have looked in depth at the research data, fundamentals, and the heart disease market. H.C. Wainwright believes a price target of USD 9 per share is realistic. They used the discounted cash flow tool to assess cash flows from 2024 to 2035, accounting for the annual number of shares to include the impacts on performance. H.C. Wainwright analyst Vernon Bernardino assumes 2% growth, with an 85% probability of treatment success for CardiolRx™.
Interim conclusion:
Due to the orphan drug status granted by the FDA, Cardiol Therapeutics could have years of exclusivity, meaning that various risk criteria no longer apply. Thanks to the impressive Phase II study results, Cardiol Therapeutics now has the potential opportunity to offer millions of people worldwide a prescribed oral medication, overshadowing the current high-cost therapies of competitors. Close collaboration with cardiologists and general practitioners is crucial for future commercialization. Prominent medical partners to date include the Cleveland Clinic, Mayo Clinic, Massachusetts General Hospital, Houston DeBakey Heart & Vascular Center, and the Tecnológico de Monterrey in Mexico. With the medical and scientific centers, Cardiol Therpeutics has good access to researching physicians internationally.
The global market for pericarditis treatment reached a value of USD 3.60 billion in 2022. It is projected to grow at an annual growth rate of 5.24% to USD 6 billion by 2032, driven by the increasing elderly population. Extensive advances in the treatment and diagnosis of cardiovascular diseases will drive the pericarditis drug market to exponential growth, with Cardiol Therapeutics being one of the key drivers. 9

This update is based on the initial report from 01/22

