
State of the art research - profile
Cardiol Therapeutics is a platform drug company developing a synthetic cannabidiol (CBD)-based liquid drug. The formulation, known as CardiolRx™, is being evaluated for the treatment of various life-threatening cardiovascular conditions. Heart failure alone costs approximately USD 30 billion annually in the U.S.
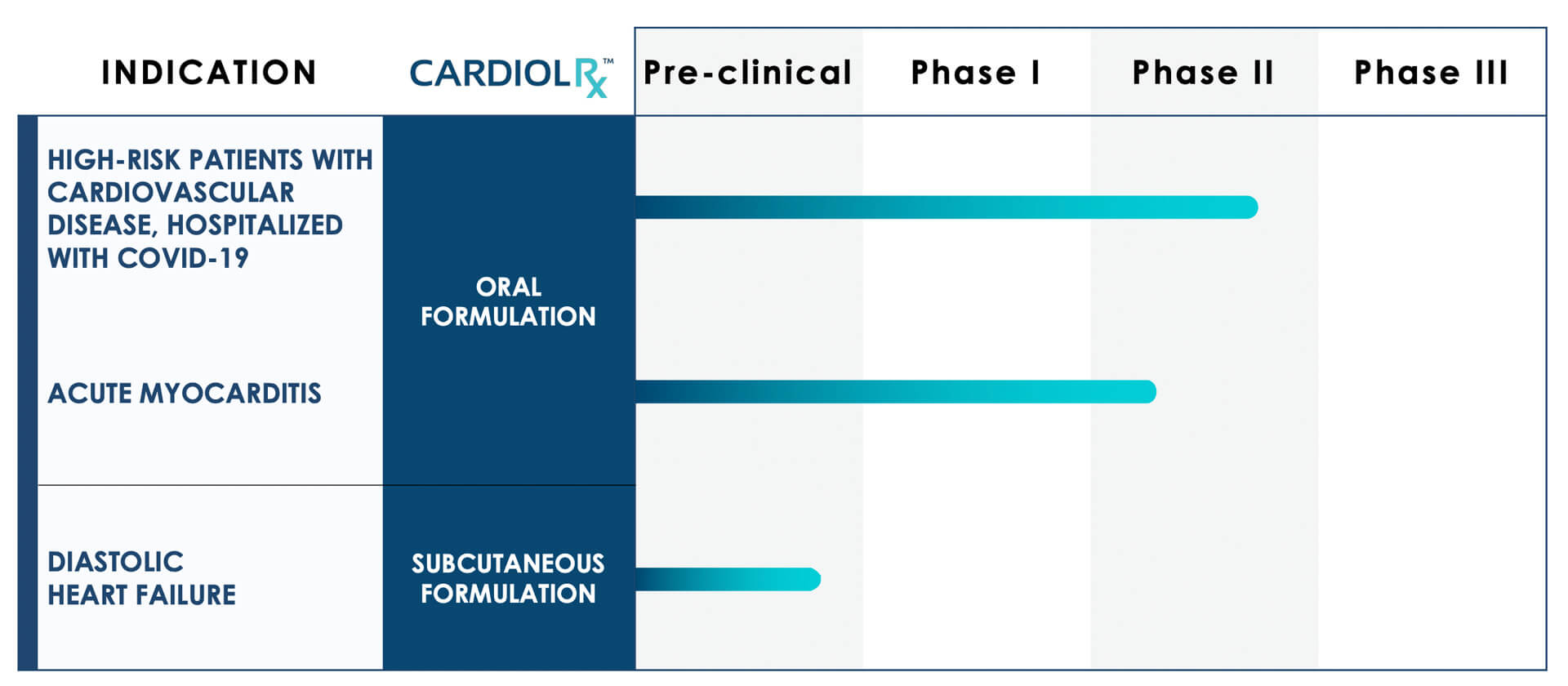
Orally administered CardiolRx is currently being investigated in a clinical Phase II/III study in patients hospitalized with COVID-19 who have a history of cardiovascular disease and/or associated risk factors."1
cannabigerolic acid and cannabidiolic acidan bind the spike protein, researchers recently found.
In addition, the company is expecting to initiate an international Phase II clinical trial of orally administered CardiolRx for the treatment of acute myocarditis. Myocarditis is a condition caused by inflammation of the heart tissue. It is a leading cause of sudden cardiac death in people under the age of 35.
CardiolRx has the same concentration but higher purity than Epidiolex®, the first FDA-approved pharmaceutical form of CBD developed as an orphan drug (extended market exclusivity of 7 to 10 years in approved markets) for the treatment of rare forms of pediatric epilepsy. From this indication, a blockbuster potential can be inferred for the Cardiol oral formulation. The third candidate currently in development is a subcutaneous (SC) formulation of CardiolRx for the treatment of chronic heart failure.
Cardiol has research collaborations with several top international centers, including Houston Methodist DeBakey Heart & Vascular Center, University of Alberta and TecSalud del Tecnológico de Monterrey, Mexico.
Cannabidiol and the impact on the cardiovascular system.
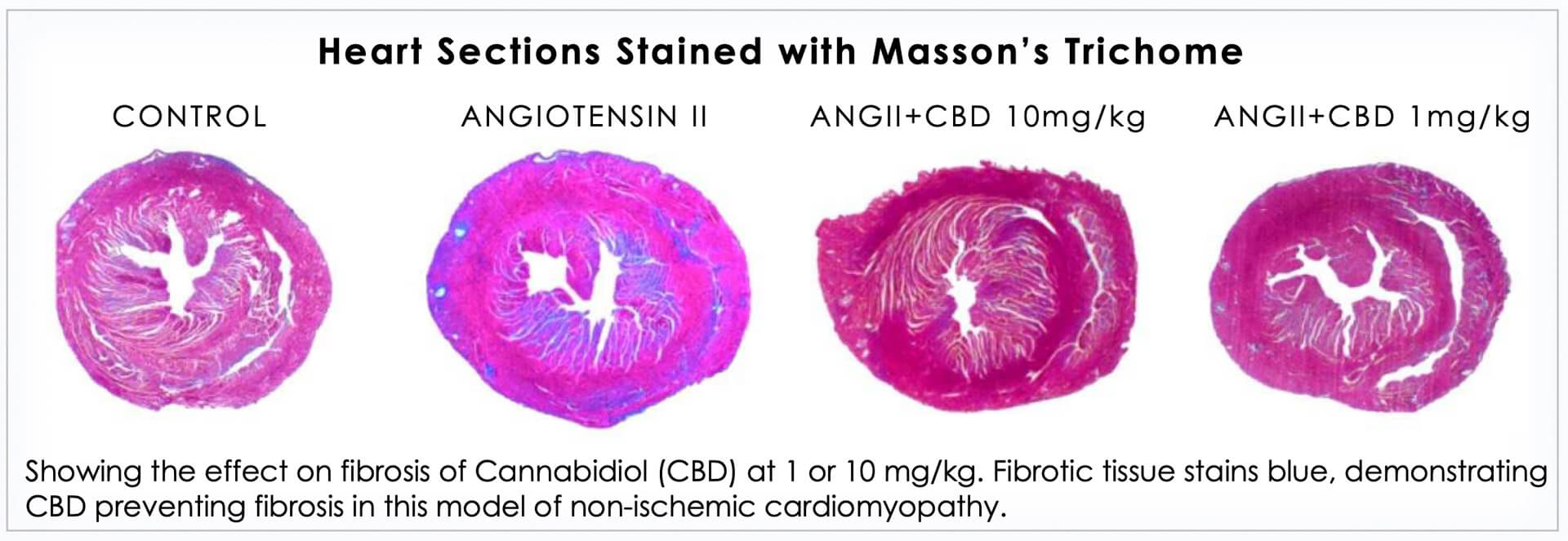
A 2016 research paper published in Molecular Medicine by WS Lee et al, which presented findings on the effect of cannabidiol on cardiac inflammation and fibrosis, demonstrated that "CBD treatment significantly attenuates autoimmune myocarditis and improves myocardial dysfunction and heart failure primarily through its anti-inflammatory and anti-fibrotic effects."
In addition, the authors concluded that "these results, combined with CBD's demonstrated safety in human clinical trials and its current FDA orphan drug approval for various neurological disorders, suggest that it has enormous therapeutic potential for the treatment of myocarditis of various etiologies and various autoimmune diseases."
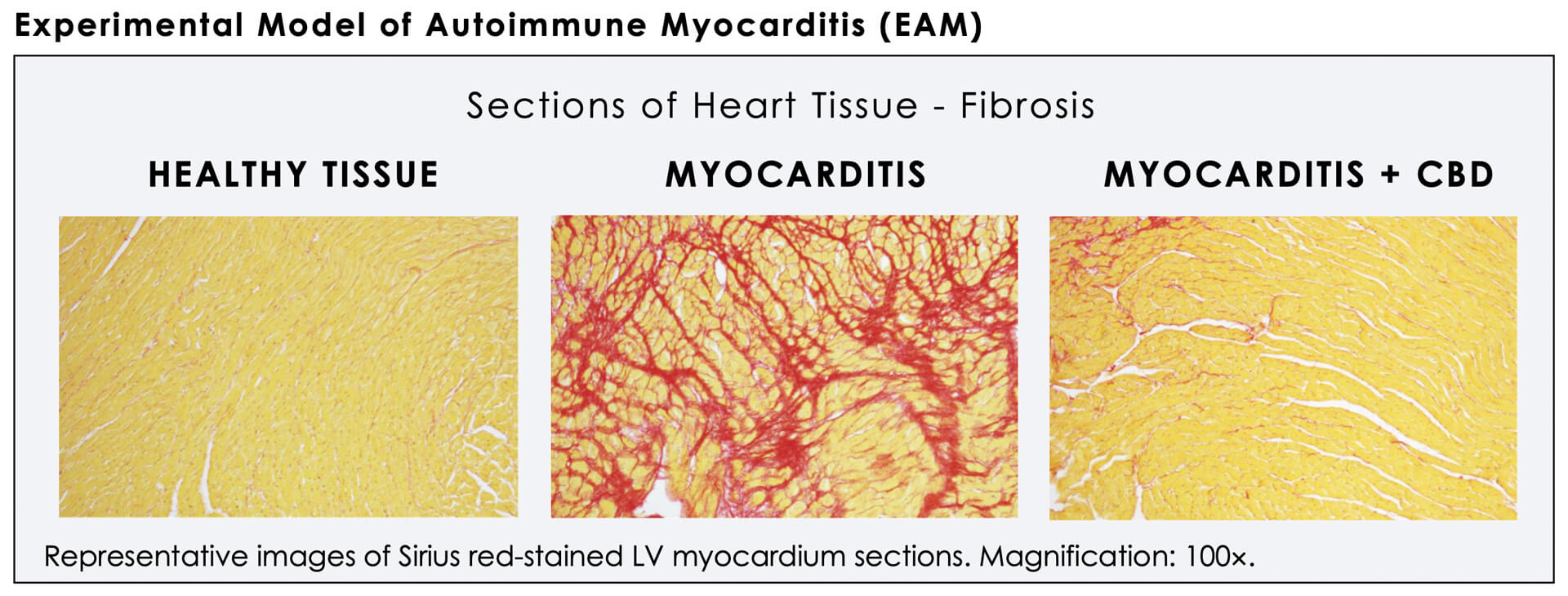
These findings formed the approach to Cardiol's business. Science is still working to understand the entire life cycle of CBD in the human body and its interaction with its various components. What is looking more and more certain however, is CBD causes vasodilatation, lowers blood pressure, and protects the heart.
Current study on Covid-19 has signal character
Ingredients can prevent infection with the virus that causes covid-19 by blocking its entry into cells.
Recently, researchers at Oregon State University published a study of great signaling character. The experts found that ingredients of cannabis prevent infection with the virus that causes Covid-19 by blocking its entry into cells.3 The researchers discovered that cannabigerolic acid and cannabidiolic acid can bind to the spike protein of SARS-CoV-2, the virus that causes covid-19. "These cannabinoids, isolated or in hemp extracts, are orally bioavailable and have a long history of safe use in humans. They have the potential to prevent and treat SARS-CoV-2 infection," the researchers said in their summary of the study.
Cannabidiol
Safety Issue #1.
Unlike other cannabinoids, CBD has an exceptional safety profile and poses no significant risk to users. To date, studies with CBD have shown none of the adverse effects associated with other cannabinoids such as THC. Compared to other cannabinoids, CBD also shows no evidence of abuse risk. Orally ingested CBD behaved like a placebo in all tests assessing dependence. Cannabidiol generally has low toxicity, no effects on most non-tumor cells, psychological or biochemical parameters.
Concentration and purity
Cannabidiol can either be derived from the cannabis plant, produced biosynthetically e.g. with yeast, or synthesized pharmaceutically. Cardiol Therapeutics uses synthesized cannabidiol. For this purpose, an exclusive supply agreement was signed with Dalton Pharma Services of Toronto (Dalton) in June 2017.
A cannabinoid from the female hemp. Anticonvulsant, anti-inflammatory, anti-anxiety and anti-nausea effects have been described.
Dalton is a leading North American cGMP pharmaceutical manufacturer. At the time of the agreement, Dalton was the only Health Canada-approved and FDA-registered company in Canada licensed to manufacture pharmaceutical cannabidiol. Dalton's unique manufacturing process allows it to produce CBD of the highest purity for highly concentrated oral formulations with virtually undetectable THC content (<10ppm) on the market.
The cannabidiol formulation produced by Dalton is characterized by two features: high concentration and high purity. This is crucial for FDA approvals. All of Cardiol Therapeutics’ formulations are based on CBD formulations produced by Dalton at a concentration of 100 mg cannabidiol per ml. Using such high concentrations can reduce stress and anxiety, has high antioxidant content, and enhance anti-arthritic and anti-inflammatory effects that can help relieve inflammatory pain.

CardiolRx™ - the foundation of Cardiol Therapeutics' pharmaceutical developments.
Cardiol Therapeutics' focus is on CardiolRx™, a highly concentrated, pharmaceutically produced cannabidiol-based therapeutic used for a variety of treatments for various cardiovascular conditions.
While development of the drug for the treatment of diastolic heart failure is still in the preclinical phase, clinical trials have begun for the other two research vectors. Phase I for the treatment of acute myocarditis has been completed. The clinical trial for the treatment of patients suffering from COVID-19 with a history or risk factors for cardiovascular disease is a potentially pivotal Phase II/III trial. Phase III trials are the final step before regulatory review and approval.
Smart business strategy
The usual pharmaceutical business model does not apply to Cardiol Therapeutics. Typically, drug development involves the discovery, development and launch steps. Cardiol Therapeutics took a different approach. The Canadians focused on the known anti-inflammatory properties of cannabidiol and skipped the entire discovery phase. The company then explored the inflammatory states of cardiac tissue and developed therapeutic candidates that can correct or offset these cardiac limitations.
This results in significant savings in time and investment costs. The company benefits from the collective efforts of the scientific community, allowing it to minimize risks and dramatically increase the chances of market readiness.

Investment Highlights
- Solid financing through capital increase of USD 50 million
- Potential takeover candidate
- Undervaluation also in terms of competition
- Analyst price targets up to CAD 17.49 per share
- Blockbuster potential of product pipeline
- Promising clinical studies
- Cannabidiol with promising cardiac and covid applications
- Large addressable markets
- Low-risk corporate strategy
- Extensive international research collaborations
Analysts see significant upside

Given the high potential and large addressable market, all four analysts attest to great upside for Cardiol’s stock. Costs related just to heart failure amount to USD 30 billion annually in the U.S. alone.
Even if Cardiol, according to the experts, should only pass the sales mark of CAD 100 million in 2027 or 2028, the share price is currently dramatically undervalued, even when compared to competitors. **The price targets of the analysts range between CAD 5.00 and CAD 15.49. with an average price target of CAD 11.00, which would be a four time increase from the current share price.
This is how much Pfizer wants to pay for the acquisition of a company in the sector.
The possibility of a takeover of Cardiol should not be forgotten. With a market capitalization of only about CAD 170 million, Cardiol is a lightweight compared to billion-dollar acquisitions made in the past. Most recently, the US corporation Pfizer swallowed the California-based Arena Pharmaceuticals for USD 6.7 billion. Arena has yet to bring a drug to market. This company has several products in clinical development in the fields of gastroenterology, dermatology and cardiovascular diseases.
Cardiol's product pipeline has blockbuster potential. The use of cannabidiol in heart disease has great potential for success. In addition, recent research suggests that active cannabinoid ingredients may prevent covid infection. Investors are still able to get exposure to this exciting company at a favorable level. Analysts believe the stock is capable of at least quadrupling. More information is expected from the company at the International Investment Forum (IIF), Feb. 17, 2022. Free to attend: ii-forum.com.

