Typically, the risk of heart disease increases with too little exercise, excessive consumption of alcohol, tobacco and an unhealthy diet. But the number of myocarditis and pericarditis cases in young people has also increased in the wake of the Corona pandemic and the measures it imposed. Strikingly, young athletes in particular 1 develop inflammation of the heart or myocardium.2
What is pericarditis?
The heart is enclosed by the pericardium (heart sac). Pericarditis is the term used when the pericardium is inflamed. Viruses are the triggers of acute pericarditis. Among the most common are enteroviruses (coxsackie and echoviruses), herpes viruses, adenoviruses, and parvovirus B19. Fungi and bacteria are rarely the causative agents.
Recurrent pericarditis is inflammation of the pericardial membrane that follows an initial episode, usually as a result of viral infection. Many patients suffer multiple recurrences associated with debilitating chest pain, shortness of breath, and fatigue. Symptoms lead to quality of life limitations, emergency room visits, and hospitalizations. Also, fluid accumulation in the pericardium or scarring can cause life-threatening heart constrictions.
To date, the only drug approved in the US is too expensive
It is estimated that 38,000 patients in the US need treatment for recurrent pericarditis each year. The only Food & Drug Association (FDA)-approved therapy for recurrent pericarditis, ARCALYST®, has been on the market since 2021 and is quite expensive at around USD 6,000 per use, with patients requiring the treatment about 4 times.3 In addition, in most cases, the cost of inpatient treatment for a period of 6-8 days is added. In the US, individuals must purchase their own health insurance. Many citizens go into debt when sick and avoid hospitalization due to cost pressures.
Kiniksa's drug ARCALYST® targets a small patient population of approximately 14,000 people per year who do not respond to regular treatments or are affected by a persistent underlying condition. Following its launch in April 2021, ARCALYST® recorded net sales of EUR 35.7 million during the year. In the fourth quarter, the drug was already generating profits; for 2022, net sales are expected to amount to EUR 111.2 million.
The market opportunity for Cardiol Therapeutics
1. Hospitals and clinics
2. Medical institutes
3. Ambulatory surgical centers
4. Research institutes
The market for pericarditis is expected to reach EUR 4.9 billion in 2030, and an estimated EUR 2.8 billion was recorded in 2021. The average growth rate is 4% between 2023 and 2030. According to open access medical journal Cureus, 4 , acute pericarditis is found in about 0.1% to 0.2% of hospitalized patients. Nearly 5% of patients affected are hospitalized for nonischemic chest pain. Pericarditis is classified into several categories: acute pericarditis, recurrent pericarditis, persistent pericarditis, and chronic constrictive pericarditis. Acute pericarditis is increasing significantly, and its symptoms include sudden heart pain.
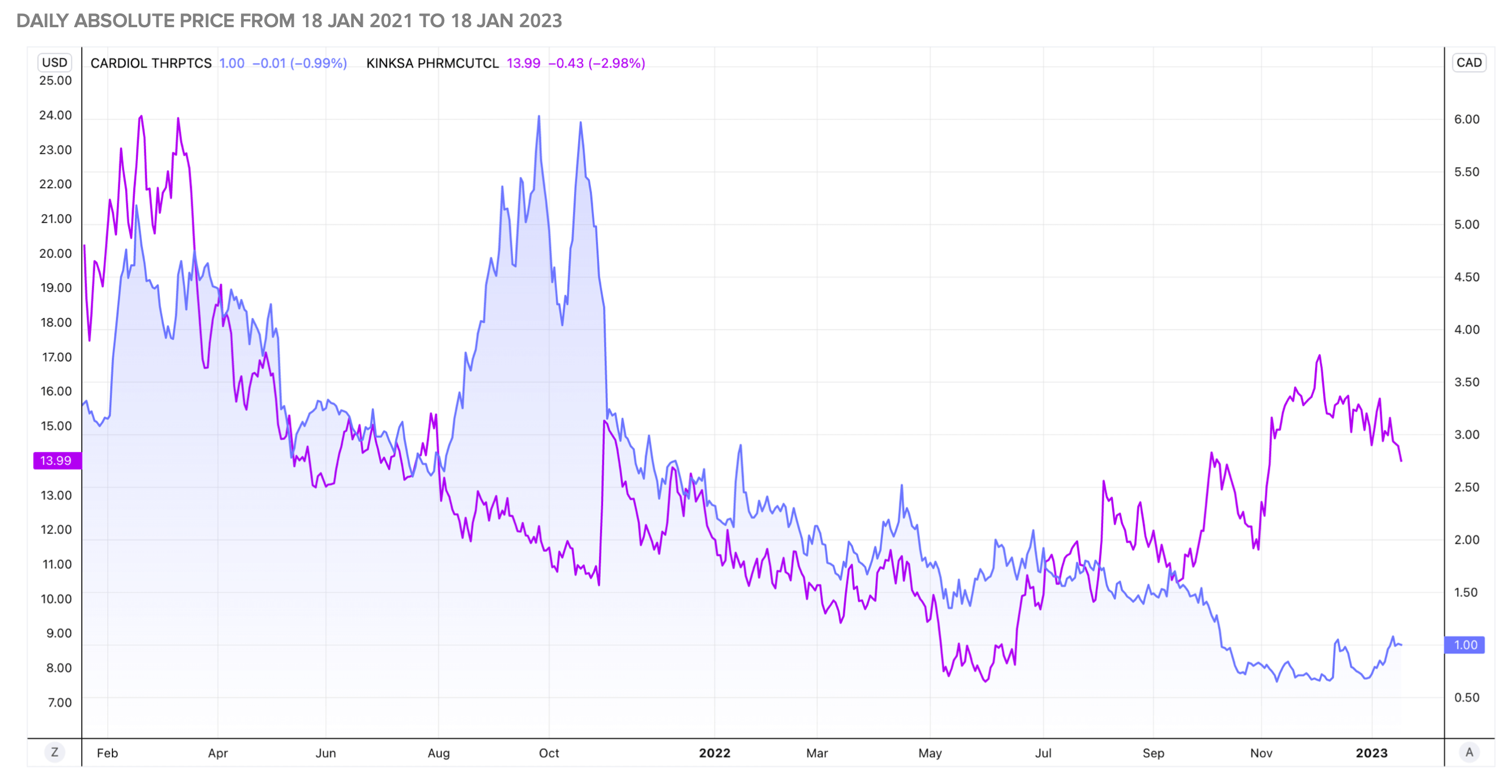
The main market competitors for Cardiol Therapeutics (ISIN CA14161Y2006) are Kiniksa Pfizer Inc, Bayer AG, PerkinElmer Inc, ALLERGEN, Lincoln Pharmaceuticals Ltd, Unicure India Pvt Ltd, Zydus Cadila Healthcare Ltd, Sun Pharmaceutical Ltd and Regeneron Pharmaceuticals Inc. 5
First patient enrolled in Phase II trial
Cardiol Therapeutics has successfully enrolled the first patient in a Phase II pilot study NCT05494788. The study's objective is to evaluate the tolerability, safety and efficacy of CardiolRx™ in patients with recurrent pericarditis. The study is designed to assess improvement in objective indicators of disease, as well as the feasibility of discontinuing concomitant treatment, including corticosteroids, for an extended period of time while taking CardiolRx™ concomitantly, in addition to the usual safety assessments.
The Phase II pilot study will enroll 25 patients at clinical centers in the US that specialize in the treatment of pericarditis. This phase of the study explores therapeutic efficacy. The protocol was developed in collaboration with leading experts in the field of pericardial disease. The study principal investigator is Allan L. Klein, MD, director of the Center for Pericardial Disease and professor of medicine at the Cleveland Clinic Heart and Vascular Institute.
The primary efficacy endpoint is the change in patient-reported pericarditis pain using an 11-point numeric rating scale ("NRS") from baseline to 8 weeks. The NRS is a validated clinical tool used in numerous conditions involving acute and chronic pain, including previous studies of recurrent pericarditis. Secondary endpoints include pain score after 26 weeks of treatment and changes in circulating levels of C-reactive protein, a commonly used clinical marker of inflammation.
At the American Heart Association Scientific Sessions 2022, preclinical data were presented by the medical scientific team, providing a solid scientific basis for the clinical investigation of CardiolRx™ in recurrent pericarditis.
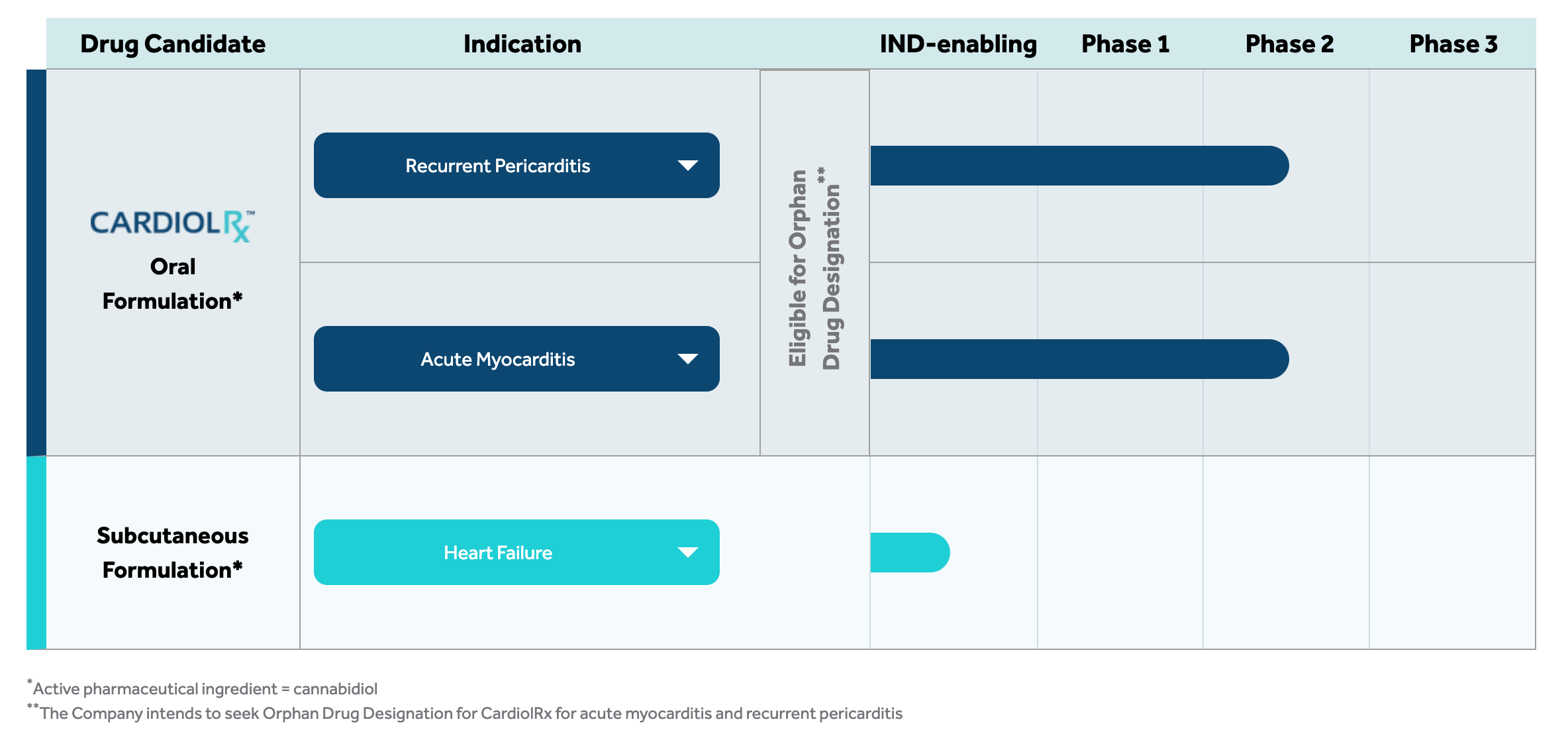
Approval for CardiolRx™ as an Orphan Drug?
Recurrent pericarditis is a so-called 'rare disease' in the US, making CardiolRx™ eligible for orphan drug designation under the FDA's Orphan Drug Designation (ODA) program. Because few people are affected by a disease, opportunities to commercialize a drug are limited and often little is known about the disease. To enable more research and development in this area, special conditions are offered, such as regulatory relief. Approved orphan drugs benefit from ten years of protection from competing products unless they prove to be more effective or tolerable.6
For investors, this means in concrete terms: The Orphan Drug Act (ODA) provides drug developers like Cardiol Therapeutics with seven years of market exclusivity. During this time, no other companies can develop generics or biosimilars, encouraging developers to profit from their unique drugs. Since the market for rare diseases is very small, such an extended exclusivity is a motivation for researchers to continue their work. In the end, otherwise overlooked patients get the benefits of the exclusivity period. Orphan drug price growth peaked in recent years but has declined slightly over the past decade. In turn, the cost of non-orphan drugs went up even more during the same period. Worldwide, investment in orphan drugs accounts for one-fifth of total drug spending.
Interim Bottom Line:
As early as 2022, Cardiol's research colleagues at Virginia Commonwealth University presented results demonstrating the protective effects of CardiolRx™ in a pericarditis model. Cardiol was founded to translate the anti-inflammatory and anti-fibrotic properties of cannabidiol into new therapeutic solutions to help people with heart disease. In addition, Cardiol is also developing a novel subcutaneously administered drug formulation of cannabidiol for use in heart failure - a leading cause of death and hospitalization in the developed world, with associated healthcare costs exceeding EUR 27.8 billion annually in the US. The Phase II trial will be followed by Phase III in the development pipeline, which will be a relatively small step to commercialization. The possibility of being introduced as an orphan drug protects against competing products.
As a prescription drug, CardiolRx™ can become a best-seller in the affordable price range, giving countless patients the quality of life they have lost suffering from recurrent pericarditis. If CardiolRx™ is successful in regulatory approval and commercialization, the Company's valuation will be impressive compared to its current market capitalization of EUR 45.4 million.
This update is based on the initial report 01/22.

