AccuTOX™ masters final step before Phase I trial
Canadian biotech Defence Therapeutics has made further progress over the past eight weeks. The Company, which offers Accum™, an adjuvant technology designed to enhance the efficacy of vaccines and other drugs, most recently reported the successful manufacture of AccuTOX™. AccuTOX™ is a chemotherapeutic based on the versatile Accum™ technology and is expected to enter a Phase I trial later this year. In order to properly submit all applications to the US Food and Drug Administration (FDA), Defence Therapeutics had already secured the services of the City of Hope University Hospital in the greater Los Angeles area months ago. With the production of drug-grade AccuTOX™, it now appears that all requirements for the start of the Phase I trial will soon be met.

(Source: Defence Therapeutics)
Drug regulatory authorities stipulate stringent manufacturing and quality control tests before any clinical trial with active ingredients. In order to meet these requirements, Defence Therapeutics has contracted Biopeptek Pharmaceuticals, a service provider, to do this preliminary work. "This is an important milestone for Defence's AccuTOX™ program. With the successful completion of manufacturing and release trials, Defence now faces the final step of filing an IND application for a Phase I clinical trial with AccuTOX™ as an injectable agent for the treatment of melanoma", commented Sébastien Plouffe, CEO and President of Defence.
City of Hope University Hospital is the perfect partner for approval
submitted by the renowned City of Hope Hospital each year.
As Defence Therapeutics states in its recent company announcement, this study is also likely to be conducted at City of Hope Hospital. The research hospital has a history of breakthroughs in treatments for cancer, HIV and diabetes and exemplifies the motto "from bench to bedside" - the latest research should directly benefit patients. Typically, the City of Hope submits around 50 applications a year to the FDA. The hospital is, therefore, a suitable partner to accompany such studies.
The active ingredient has already cleared the first hurdle for the approval of AccuTOX™ as the subject of a Phase I study. AccuTOX™ was exposed to temperature fluctuations, light and humidity. The tests showed that the active ingredient is stable at temperatures ranging from -20 °C to 5 °C. Thus, AccuTOX™ meets all stability criteria for peptide-active ingredients used in clinical trials.
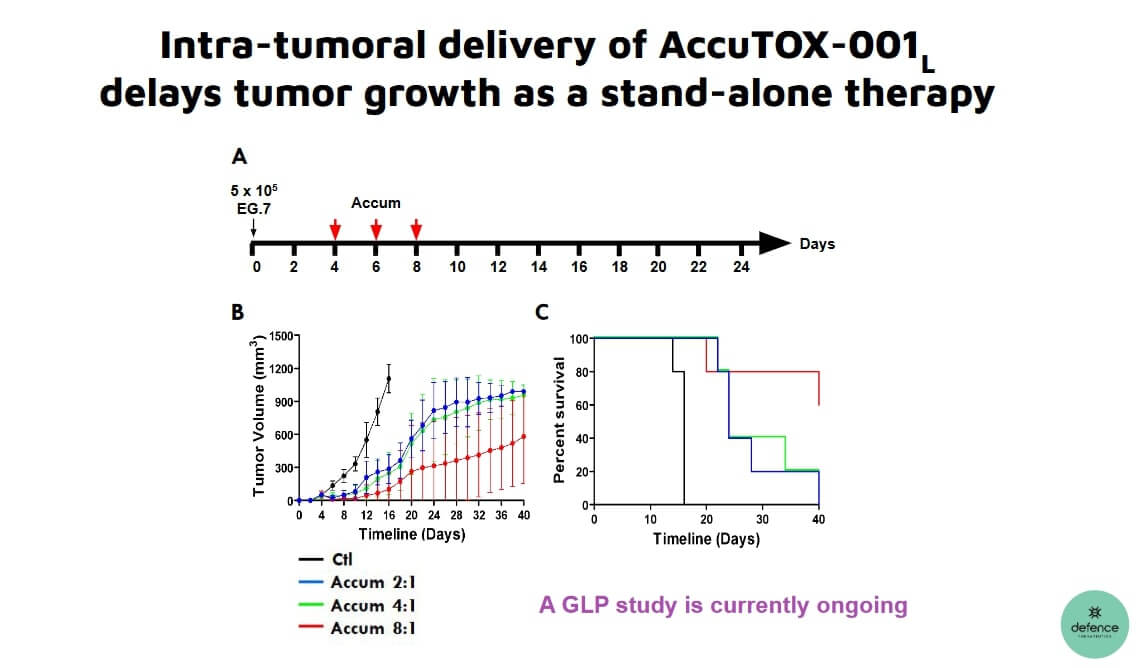
ARM vaccine with 100% efficacy - will breakthrough in pancreatic cancer now follow?
The latest announcement from Defence Therapeutics joins a list of other advances the Company has made in recent weeks. For example, it completed a preclinical study in animals that showed 100% efficacy in treating lymphoma. This preliminary work is also expected to lead to a clinical trial soon. "These trial runs and all required quality control steps are central to obtaining approval for our Phase I solid tumor trial. This promising therapeutic against already established lymphomas is cost-efficient and easy to manufacture. Defence's A1-2 dimer has excellent therapeutic efficacy," adds Dr. Moutih Rafei, Vice President Research and Development at Defence Therapeutics. Because of the vaccine's versatility and success in lymphoma and melanoma, Defence Therapeutics hired a service provider about two weeks ago to test the vaccine for efficacy in pancreatic cancer. If this test is successful, Defence Therapeutics' ARM vaccine could also become a beacon of hope for other difficult-to-cure cancer variants.
Extensive patent protection for Accum™ key to collaborations
It appears that AccuTOX™ may become Defence Therapeutics' first compound to move into a Phase I trial. Other compounds and procedures are likely to follow. Meanwhile, Defence Therapeutics has obtained broad patent protection for its Accum™ technology from the US Patent Office. The patent protects Accum™ as a drop-in technology that enhances the immunogenicity and performance of virtually any cell- or protein-based vaccine and covers both prophylactic and therapeutic vaccines against cancer and infectious diseases. Defence Therapeutics rates the rapid patent award as evidence of the solid scientific foundation of its technology. The patent is intended to be the basis for protecting the technology as globally as possible. This would also open up the possibility of licensing Accum™ for other uses, and in this way, either generate cash flows or attract partners to explore further opportunities around the technology.
Defence Therapeutics is advancing a protein-based cervical cancer vaccine for both prophylactic and therapeutic use, in addition to its ARM solid tumor cancer vaccine and AccuTOX™ chemotherapeutic agent for melanoma, breast and lung cancer. Furthermore, the Company is working on mRNA vaccines for various indications. A study is currently underway to demonstrate the benefits of Accum™ in combination with mRNA vaccines. Results are expected in a timely manner. In particular, mRNA technology is seen as a hopeful candidate due to its versatility and ease of use. In particular, mRNA vaccines benefit greatly from processes that increase their efficacy. For example, the success of BioNTech's corona vaccine Comirnaty is also based on such a process. Accum™ could still have great potential here after achieving worldwide patent protection and complement existing technologies to increase efficacy. At the same time, Defence Therapeutics could be ahead of the competition in the development of new mRNA vaccines thanks to Accum™. In late April, Defence Therapeutics hinted at working on a vaccine against a potentially deadly disease. Details will be released as the project progresses.
Bottom line: When versatility meets low valuations
The share of Defence Therapeutics has come back after a furious start to 2023. The recession fears and interest rate worries have weighed on the share prices of growth companies in the biotech sector. However, for investors who see Defence Therapeutics as a promising company, the current price level could be extremely attractive. The Company is moving forward unwaveringly with the development of its numerous projects. While AccuTOX™ and the ARM vaccine are about to enter Phase I trials, Defence is testing its compounds in an increasing number of disease indications. In particular, testing of the ARM vaccine in hard-to-cure cancer variants such as pancreatic cancer is likely to be watched warily within the biotech community. Given the versatility of Accum™ and the progressing patent protection, it is not impossible that the Company, which is currently valued at approximately CAD 118 million, will attract the attention of major players in the biotech and pharmaceutical sectors.
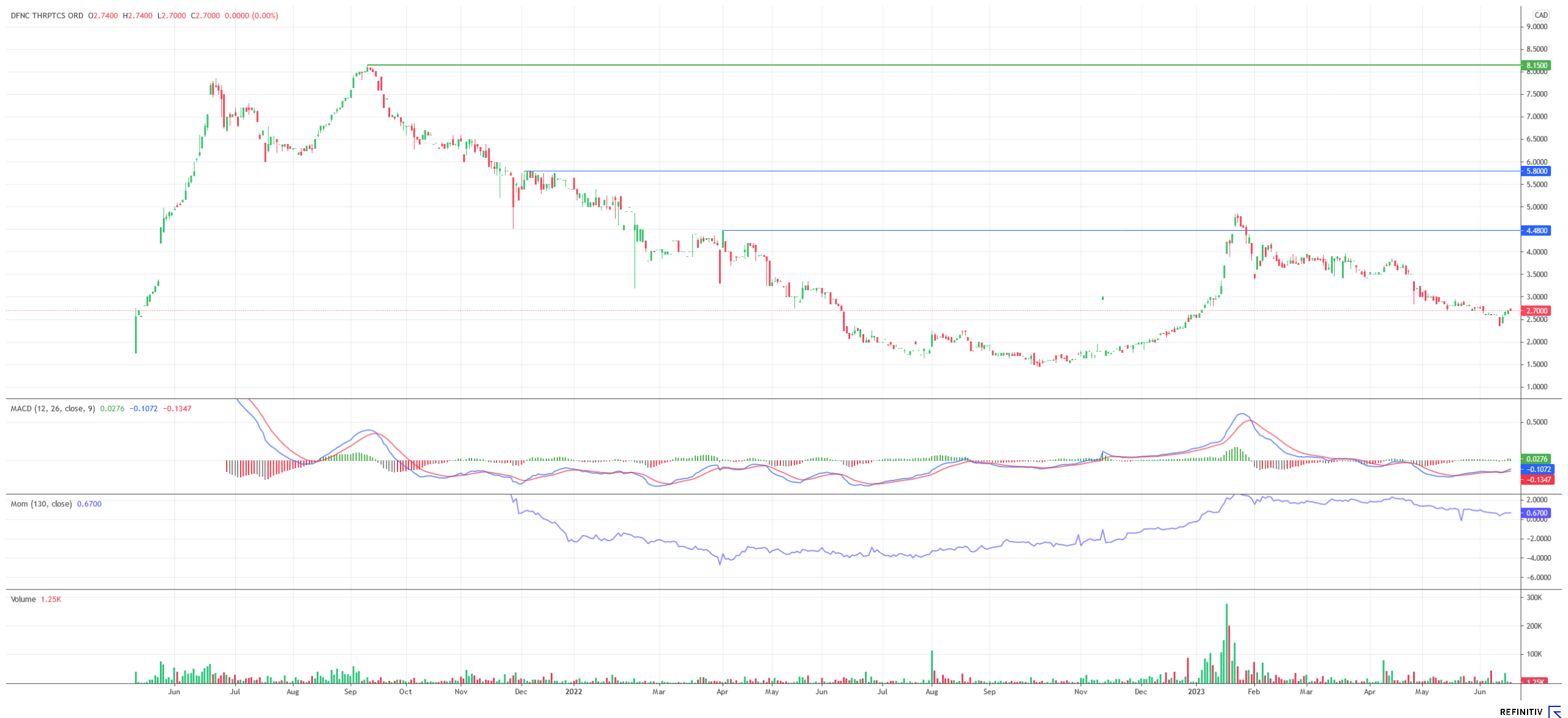
The update is based on the initial report 12/2021

