Accum™: Comprehensive patent protection filed in 157 countries
Canadian biotech Defence Therapeutics is expanding its patent portfolio. The cancer specialist has filed for worldwide protection of its Accum™ technology for intracellular delivery of various drugs and vaccines, including polynucleotides, recombinant proteins and nucleoprotein complexes under PCT standard. This is an international intellectual property treaty that ultimately results in national patents in all member states. There are 157 countries worldwide that are party to the PCT treaty. But what exactly does the patent cover? What are polynucleotides or nucleoprotein complexes?
received the Nobel Prize in Chemistry in 2019
In the broadest sense, these are chains of amino acids that make up genetic information, such as RNA or DNA. They also include proteins that can bind to RNA or DNA. The patent filed by Defence Therapeutics thus covers the property of Accum™ as a drug enhancer of mRNA vaccines as well as the property as a carrier of processes such as CRISPR. CRISPR is a process that is also called "gene scissors". It makes it possible to remove damaged genetic information from existing DNA and replace it with new information. The process works in principle in all organisms and led to the Nobel Prize in Chemistry in 2020 for the two discoverers, Jennifer Doudna and Emmanuelle Charpentier.
"Gene scissors" CRISPR as a further argument for Accum™
CRISPR is not yet used clinically, but it is already standard in in vitro research. In the CRISPR process, an RNA that behaves complementarily to the target gene is coupled with an enzyme. The RNA "chooses" its target and brings the enzyme, i.e. the actual "gene scissors", to the target. First studies in patients with Duchenne muscular dystrophy already showed promising results. After CRISPR treatment, patients showed increased levels of a protein that cannot actually be produced in this disease due to the existing gene defect. Cancer and HIV patients have also been the subject of initial clinical trials with CRISPR.
is expected to grow by 9.9% p.a. until 2028.
Despite the progress the technique is making in various projects, transporting the "gene scissors" inside the cell is still considered a major challenge. Currently, the most common way is to use inactivated viruses as transport carriers. Nanoparticles, such as liposomes, can also protect the "gene scissors". However, all methods are considered error-prone and, like nanoparticles, are not yet mature enough. This is where Accum™ technology comes in. As is so often the case in biology, the combination of multiple assistive technologies could ultimately lead to an increase in the effectiveness of biotechnological processes, such as CRISPR. According to Precision Reports market researchers, the CRISPR applications market was worth USD 1.07 billion in 2022 and is expected to grow by 9.9% annually to USD 1.89 billion by 2028.
ARM vaccine as a great hope against various types of cancer
Although using the "gene scissors" in clinical therapies is still a long way off, Defence Therapeutics' patent application highlights the versatility of Accum™ as a drug enhancer for various agents and vaccines. In particular, the role of Accum™ as an adjuvant technology in mRNA vaccines is likely to be highly relevant for Defence Therapeutics. mRNA vaccines have become socially acceptable since the pandemic and allow flexible adaptation of vaccines to different disease patterns. Especially in connection with cancer, mRNA vaccines are seen as great hope. Currently, Defence Therapeutics is testing the efficacy of Accum™ in the context of mRNA vaccines and aims to prove that its technology can improve the effectiveness of these vaccines. The Company has announced results for the upcoming weeks.
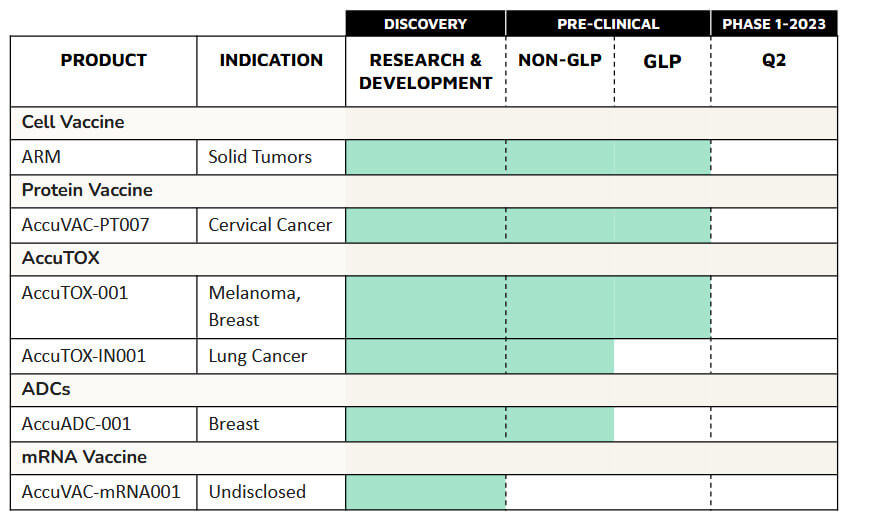
The filed patent also includes in vivo data on the efficacy of Accum™ in the context of converting mesenchymal stem cells into efficient antigen-presenting and immunostimulatory cells. Defence Therapeutics has taken advantage of this property in the development of the second generation of its ARM vaccine, which showed 100% efficacy in established lymphomas in a preclinical study in animals in late May. The results in lymphoma and melanoma were so promising for Defence Therapeutics that the Company plans to test its ARM vaccine in pancreatic cancer. Defence Therapeutics expects this application in hard-to-cure cancers to have a signaling effect and provide new insights into potential additional applications.
Is the US interest rate pause rekindling takeover fever?
"The filing and publication of this new patent application is another important step towards the commercialization and approval of the innovative therapeutic and prophylactic candidates currently in Defence's arsenal. The newly published patent application complements Defence's rapidly growing portfolio as the Defence team continues to work towards the goal of bringing its innovative therapeutic technologies to market and sustainably improving the lives of patients worldwide", said Sébastien Plouffe, CEO and President of Defence Therapeutics.

The Company's operational progress, particularly the comprehensive patent protection it seeks, could come at the right time for Defence. The Nasdaq Biotechnology Index recently rallied and is currently in an interesting situation from a chart perspective. Biotech companies also remain the focus of attention for large pharmaceutical companies. After the acquisition of the cancer specialist Seagen by Pfizer for USD 43 billion, analysts expect further acquisitions in 2023. Back in January, analysts at Canaccord Genuity, in the person of John Newman, gave hope to shareholders of companies like Defence Therapeutics: "We continue to expect a biotech bull market in 2023, which could develop into an acquisition wave if credit conditions improve," Newman told North American media at the time. The analyst expects that 2023 may see an increase in primarily smaller acquisitions against cash bids ranging from USD 1 billion to USD 10 billion. After the Fed's interest rate pause big players in the pharmaceutical industry, whose "war chests" are often well-filled after the pandemic, may consider pulling out their plans again.
Conclusion: The best is yet to come
The international patent application now filed underscores the ambitious goals that Defence Therapeutics has with its Accum™ technology. Comprehensive patent protection is also a prerequisite for either licensing the technology to other companies in return for fees or rolling it out to new fields of application as part of collaborations. Also, with valid patents, Defence Therapeutics' extensive application portfolio receives a "price tag" - which can only be good news for shareholders. In addition, there are the impending Phase I studies around AccuTOX™ - for the Phase I study of the chemotherapeutic agent, Defence won the renowned City of Hope hospital in the greater Los Angeles area just a few months ago - and the Phase I study for the ARM vaccine against lymphoma. The results of the comparative study between mRNA vaccine and mRNA vaccine in combination with Accum™ could also create a sensation. Since the stock has meanwhile also set the signs for a comeback in chart terms, the coming weeks should be particularly exciting for Defence Therapeutics.
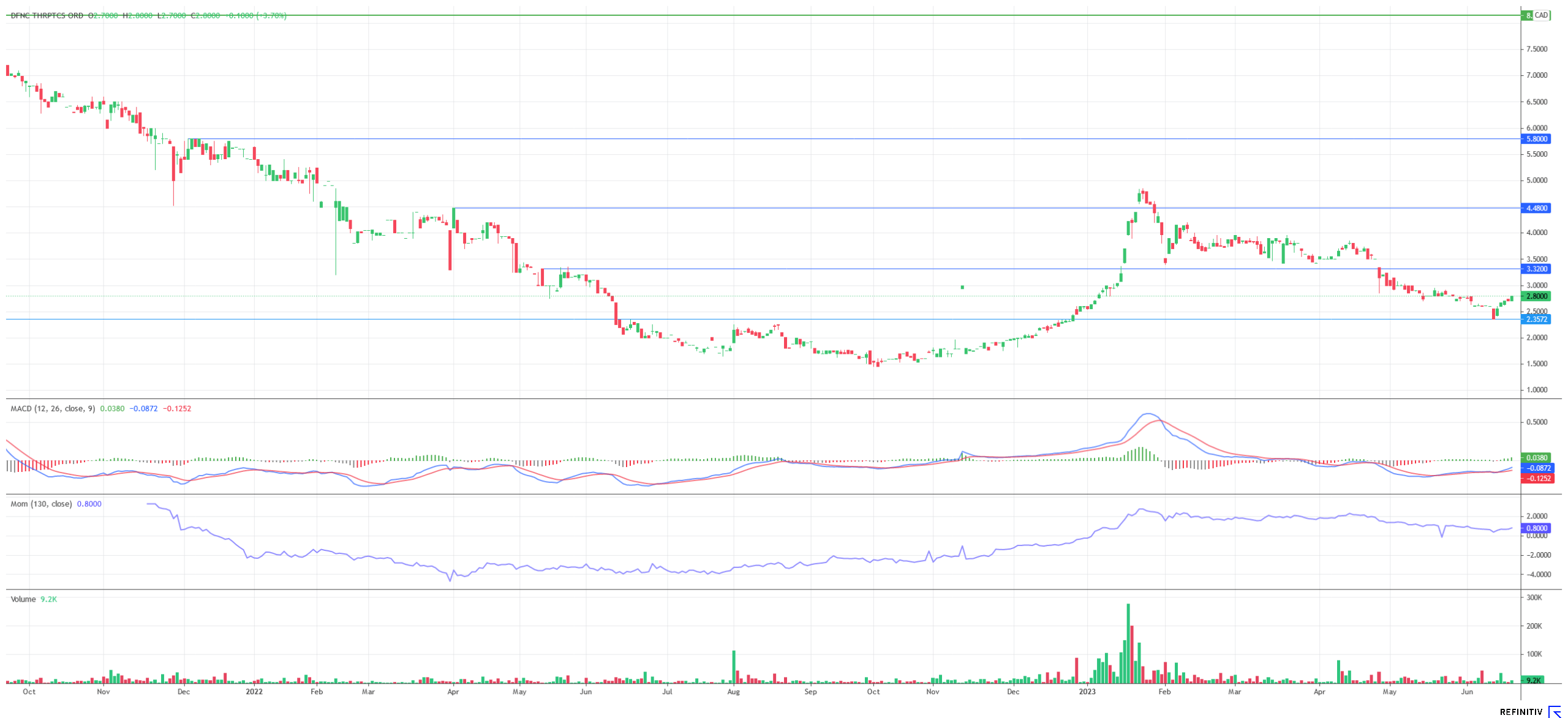
The update is based on the initial report 12/2021

