Study designs show remarkable parallels - mRNA breakthrough in 6 weeks?
The recently released news is specifically about treating melanoma in a Phase 2b clinical trial. The study involved 157 patients with stage III and IV skin cancer. After the tumor was surgically removed in each case, an mRNA vaccine was administered that was encoded for 34 so-called neo-antigens of the individual tumor. In combination with the mRNA vaccine, the checkpoint inhibitor pembrolizumab was used. The comparison group was treated with pembrolizumab alone. The risk of recurrence and death decreased by 40% with the combination of mRNA vaccine and checkpoint inhibitor. Defence Therapeutics is also currently running a pure-form mRNA vaccine against the combination of mRNA vaccine and Defence Therapeutics' patented Accum™ technology.
The basic assumption behind it: Accum™ can help biological agents of different compositions overcome biochemical hurdles within cells. This can increase the efficacy and safety of drugs. The first part of the recently launched comparative study could deliver initial results in less than six weeks, showing that the combination of mRNA vaccine and Accum™ provides higher antibody concentrations.
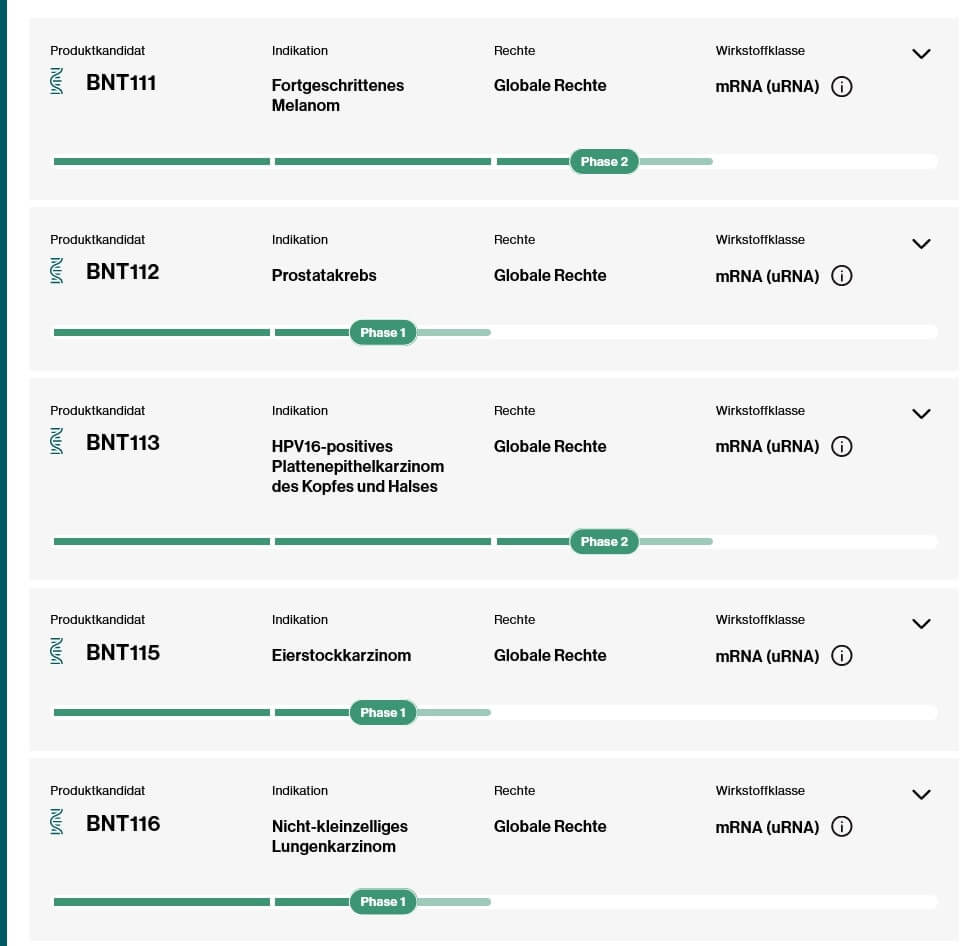
A similar thought process is behind Moderna's recent study results related to skin cancer. Experts have assumed for years that immunotherapies, such as the immune checkpoint inhibitor pembrolizumab used in the study, work even better when combined with additional treatments - the good results of mRNA vaccine in combination with pembrolizumab are clear. BioNTech is also focusing on complementary technologies around mRNA therapy. The product candidate BNT113, which targets tumors of the head and neck triggered by human papillomavirus 16, also relies on pembrolizumab in addition to an mRNA chemically modified using pseudouridine: the trial involves therapy with the immune checkpoint inhibitor alone on the one hand and combination with the mRNA vaccine on the other. The study design around product candidate BNT111, which is being investigated in phase II against advanced melanoma, is similar. Here, only cemiplimab, a product of Regeneron and Sanofi, is used as a PD1 inhibitor.
Research indicates: Is Accum™ the long-awaited multi-tool?
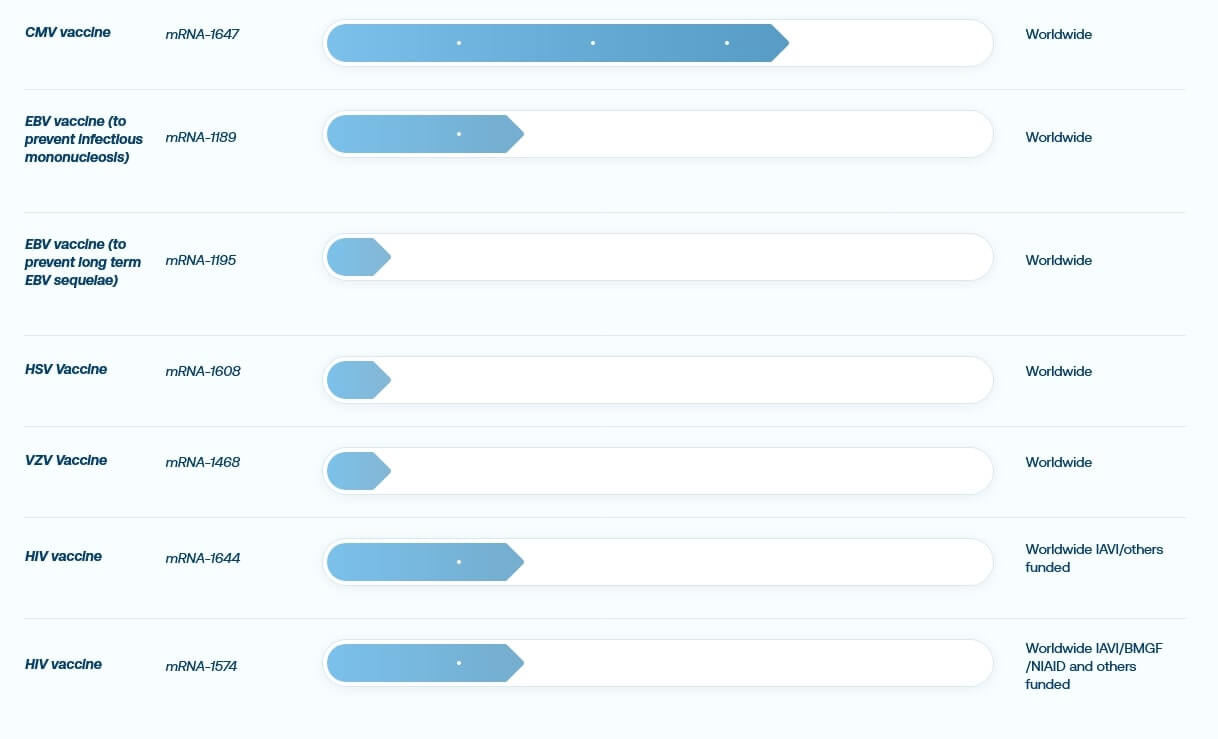
Looking at the product pipelines of Moderna and BioNTech, there are numerous projects around cancer but also infectious diseases such as RSV, CMV, EBV and HIV. The study design already underlines the current consensus in science that it is often the combination of different approaches and the use of auxiliary technology that ultimately leads to success. In addition to mRNA technology, BioNTech, for example, also relies on cell therapies and antibodies. The product candidate BNT411, a so-called small molecule immunomodulator, addresses intracellular tumor targets to stimulate a wide range of immune cells. The ultimate goal: improve treatment standards of cancer therapies. So BioNTech, too, has recognized that, ultimately, it is all about efficiency.
A look at the activities of renowned biotech companies such as BioNTech and Moderna shows that Defense Therapeutics, with its Accum™ technology, has adopted an approach that the big players in the industry are also taking: Any action to improve the effectiveness of biologic agents is welcome. To date, Defense Therapeutics believes that Accum™ can play to its strengths in combination with various active ingredients. In connection with the ARM cell vaccine against solid tumors, the Company is already immediately approaching a human clinical trial. The protein vaccine combined with Accum™ against cervical cancer caused by the HP virus is also already at an advanced stage. In six weeks, Defence Therapeutics' mRNA project could also be similarly advanced.
Is successful mRNA trial attracting potential buyers?
"Although a promising technology, mRNA vaccines have not yet reached their full potential. By conjugating mRNA with Accum™, we expect to improve the immunogenicity of the vaccine, leading to a strong immune response."
If the currently ongoing mRNA studies are completed successfully, Defence Therapeutics' activities will likely attract the attention of other companies in the industry. In the area of nuclear medicine, there is already a cooperation with the French state-owned Orano. As the development pipelines of Moderna and BioNTech show, there are numerous potential points of contact with Defence Therapeutics. In addition to collaboration in the field of mRNA vaccines, the use of Accum™ is also conceivable in principle for cell therapies and antibodies. Although such considerations must first be confirmed in studies, experts believe there is little to prevent Accum™ from playing to its strengths in these areas.
Interim conclusion: Effectiveness as the key to effective therapies
Skin cancer, cervical cancer, breast cancer: Defence Therapeutics operates in the same areas as many large biotech companies. Like Moderna and BioNTech, Defence Therapeutics believes that it takes adjuvant technologies and other tricks to increase the effectiveness of biologics. **Defence Therapeutics has been working on this assumption for years and could soon reap the fruits of its labor. Phase I trials of the cell vaccine ARM and AccuTOX, the chemotherapeutic agent consisting of a highly potentized version of Accum™, are conceivable before the end of the year.
The recently initiated studies on the interaction of Accum™ and mRNA vaccines could help Defence Therapeutics achieve a breakthrough. The race by major biotech companies such as BioNTech and Moderna around vaccines against widespread cancers, such as skin cancer, could soon shift the focus to innovative adjuvant technologies, such as Accum™. Since the drug enhancer is designed to help all biological active ingredients, there could also be further contact points. With a market capitalization in the low triple-digit million range, Defence Therapeutics remains an exciting option for risk-conscious investors.
The update is based on the initial report 12/2021

