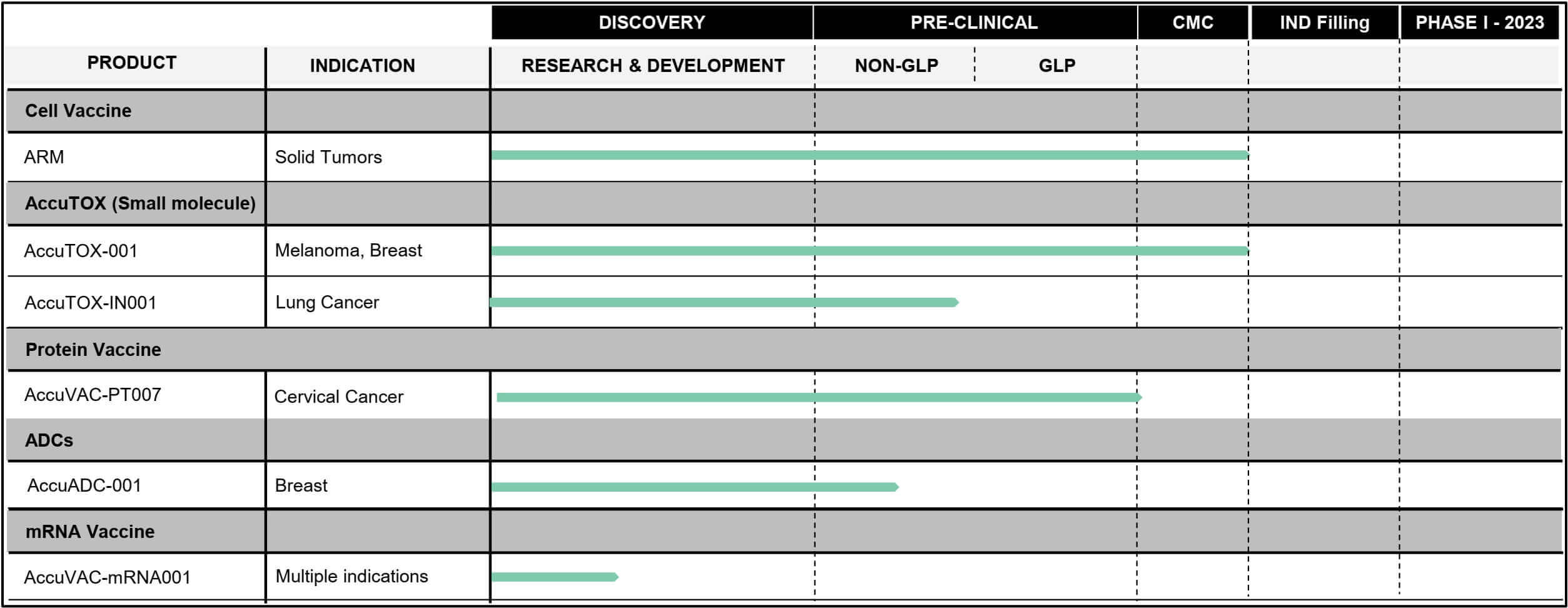
Accum™ aims to make mRNA vaccines better
the market for mRNA therapeutics is expected to grow until 2030.
The effect of mRNA on the body is also expected to be enhanced thanks to Accum™. "Although a promising technology, mRNA vaccines have yet to reach their full potential. By conjugating mRNA with Accum™, we expect to enhance the immunogenicity of the vaccine, leading to a strong immune response," said Dr. Moutih Rafei, VP of Research and Development at Defence Therapeutics. Whether Accum™ mRNA vaccines do make vaccines better is now to be revealed in vivo studies. First, the comparison between the mRNA-Accum™ combination with naked mRNA will start on solid T-cell lymphomas and then on additional solid cancer tumors. To this end, Defence Therapeutics plans to manufacture its own Accum™ mRNA vaccines in each case. The Company has already made the necessary preparations to adapt the specific mRNA to new cancer types.
Defence Therapeutics is implementing plans step by step
Defence Therapeutics is now seeking partnerships with companies already researching mRNA vaccines and sees major opportunities in immuno-oncology and infectious diseases. According to market researchers at Precedence Research, the market for mRNA therapeutics is expected to exceed USD 128.14 billion by 2030 and grow by approximately 13% annually from 2022 to 2030.
The now-reported launch of the mRNA vaccine program against cancer had been hinted at in recent months. The Company had already communicated that combining mRNA vaccines with Accum™ technology makes sense and how Accum™ efficacy can be scientifically proven. The announcement again underscores how flexible Accum™ technology is and, probably more importantly for investors, that Defence Therapeutics is capable of pursuing the many options Accum™ offers in parallel.
From Accum™ to AccuTOX™: Expert approval is high
A few weeks ago, the Company announced a cooperation between Defence Therapeutics and the French state-owned Orano. Specifically, this involves using Defence Therapeutics' patented Accum™ technology as a radionuclide-antibody conjugate. This involves a carrier technology, such as Accum™, delivering a radioactive emitter near the nucleus of tumor cells instead of a drug. The more reliably this is done, the higher the chance that the radioactive emitter will damage the cancer cell's nucleus and thus induce cell death.
Accum™ is also said to be exceedingly potent as a drug enhancer around other active ingredients, such as proteins. In addition, Accum™ itself in potentized form is said to be capable of acting as a chemotherapeutic agent. This form of the "multi-tool" patented by Defence is called AccuTOX™. A few weeks ago, the Company also announced an exciting cooperation: The renowned "City of Hope" hospital in the greater Los Angeles area is to support the application process for a Phase I clinical trial with AccuTOX™. The hospital is one of the largest university hospitals in the USA and a partner of numerous biotech and pharmaceutical companies. If this is successful, it would be another accolade for Defence Therapeutics from a renowned specialist institution. As recently as February, the Company received approximately CAD 600,000 in funding from the Canadian Biotech Incubator CQDM. This organization is considered a catalyst for biotech projects and is supported by leading pharmaceutical companies**, including Pfizer, Merck, GlaxoSmithKline, Roche, Boehringer Ingelheim, Janssen, Eli Lilly Canada, Novartis Pharma Canada, Servier, Sanofi Canada, Takeda, AstraZeneca and Amgen, in addition to government organizations.
Billion-dollar market: Will Accum™ become the mRNA problem solver?
In view of already established collaborations with renowned companies, university hospitals and other institutions, such as the Canadian CQDM, it is obvious that the combination of Accum™ with mRNA vaccines can also be a success. However, only the comparative studies already announced by Defence Therapeutics can provide concrete results. It is clear from the successes already achieved to date that the Company can score points with its technology, particularly around mRNA. When, after the outbreak of the pandemic, companies such as BioNTech and also CureVac sought a COVID-9 vaccine, the efficacy of the new mRNA vaccines differed, in some cases considerably. While BioNTech succeeded, CureVac failed across the board. In the next few days, we will explain in a "deep-dive" report how Accum™ aims to improve mRNA vaccines, where companies like CureVac once failed, and what other options there are to prevent adverse reactions to the administration of mRNA.
Interim Conclusion
Several potential blockbuster projects meet an attractive valuation
The recent company news surrounding the launch of the mRNA vaccine program against cancer shows that several projects go hand in hand at Defence Therapeutics and that the Company manages to pursue even ambitious goals in parallel. Now clinical trials will have to show whether Accum™ is capable of making mRNA vaccines even better, as Defence's head of research Rafei expects. We will shed light on the potential that could emerge if this succeeds in another article in the coming days. Given the numerous parallel projects and versatility of Accum™, the stock is promising.
Also, the market capitalization in the low triple-digit million range leaves room for a new share price increase after weeks of consolidation. A detailed analysis of the challenges associated with mRNA vaccines and the role that Accum™ could play in the future as a problem solver for such vaccines is required to conclusively evaluate the news from Defence Therapeutics.
The update is based on the initial Report 12/2021

